A brand new Diels-Alderase, CtdP, makes use of a novel mechanism to prime the substrate substance whereas changing nicotinamide adenine dinucleotide phosphate, or NADP+, into its decreased type, or NADPH, enabling the Diels-Alder response, a broadly used methodology of synthesizing necessary supplies and prescription drugs. Credit: Gao lab/Rice University
Just as a choreographer’s notation tells a dancer to strike a selected pose, an enzyme newly found by Rice University scientists is ready to inform particular molecules exactly the way to organize themselves, right down to the angle of single hydrogen bonds.
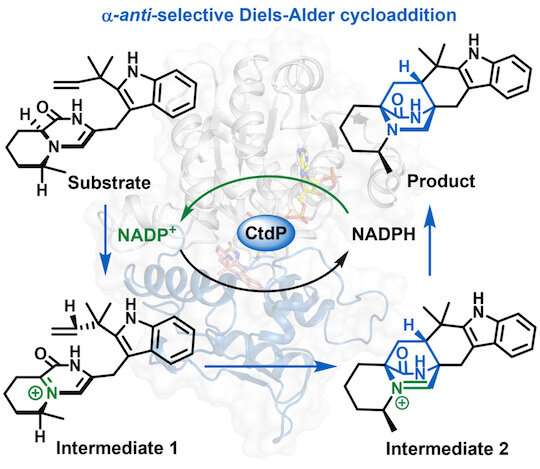
Biomolecular engineers at Rice recognized a brand new Diels-Alderase (DAase), an enzyme that catalyzes the Diels-Alder response, a broadly used methodology of synthesizing necessary supplies and prescription drugs, from uncooked supplies for plastics and fuels to artificial steroids.
The enzyme, often called CtdP, was beforehand regarded as a distinct sort of protein—a “regulator” controlling for gene expression. Regulators sometimes don’t serve a catalytic operate, that means they can’t “rework compound A into compound B,” stated examine co-author Xue Sherry Gao.
The precision of CtdP’s catalytic exercise is notable. Gao’s staff was in a position to pinpoint a minute distinction in a molecule’s spatial construction—or stereochemistry—as straight brought on by the newly recognized enzyme. “Only when our DAase is added do you get that very particular stereochemistry,” Gao stated. “This is essential, as a result of a delicate change within the stereochemistry of a small molecule can spell the distinction between a drug and a poison.”
The examine is printed in Nature Chemistry.
First described by Kurt Alder and Otto Diels in 1928, the Diels-Alder response generated a variety of business and pharmaceutical purposes, incomes the 2…
2023-01-23 11:28:04 New enzyme might imply higher medicine
Source from phys.org