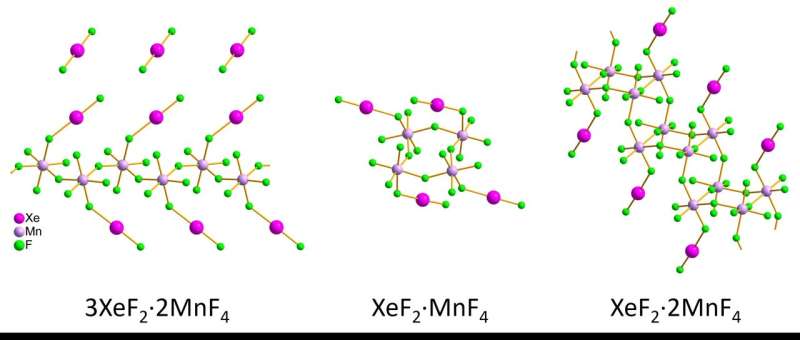
The intricate structures of three xenon compounds were effectively identified through the innovative technique of 3D electron diffraction. This breakthrough, credited to Matic Lozinšek, sheds light on the elusive nature of noble gas compounds.
Noble gases, often perceived as unreactive elements, were first bonded by Neil Bartlett over six decades ago with the creation of XePtF6. Despite this milestone, the challenge of growing large crystals containing noble gases has hindered a comprehensive understanding of their structures and functions. However, recent advancements have allowed researchers to explore the microscopic world of noble gas compounds.
The utilization of 3D electron diffraction has provided a new perspective on the structures of nanoscale crystallites, offering stability in air-sensitive compounds. By synthesizing xenon difluoride–manganese tetrafluoride compounds, researchers were able to analyze the bond lengths and angles within these tiny crystals. The comparison between 3D electron diffraction and single-crystal X-ray diffraction revealed promising results, showcasing the potential of this cutting-edge technique.
This groundbreaking research, published in ACS Central Science, marks a significant step towards unraveling the mysteries of noble gas compounds. The collaboration between scientists like Lukáš Palatinus and Matic Lozinšek highlights the importance of innovative approaches in scientific exploration.
2024-08-14 07:15:02
Original from phys.org