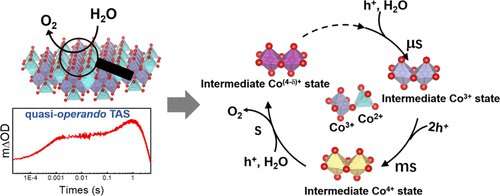
Graphical abstract. Credit: Journal of the American Chemical Society (2023). DOI: 10.1021/jacs.2c11508
Catalytic oxygen evolution reaction (OER) plays an important role in clean energy storage and conversion processes. Its transformation requires sequential intermediate valence change steps, which makes the dynamics of the catalytical cycle complicated.
Such a multi-redox mechanism involving multi-sites has implications to dictate the catalytic water oxidation. However, understanding the sequential dynamics of multi-steps in OER cycles on catalysts is still challenging.
Recently, a research group led by Profs. Li Can and Wang Xiuli from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has revealed sequential oxidation kinetics and determining roles of multi-cobalt active sites on Co3O4 catalyst for water oxidation.
The study was published in Journal of the American Chemical Society on Feb. 1.
The researchers employed quasi-operando transient absorption spectroscopy to study a typical photosensitization strategy, using Co3O4 nanoparticles as model catalysts.
They found that when OER was initiated from fast oxidation of surface Co2+ ions, both surface Co2+ and Co3+ ions were active sites of the multi-cobalt centers for water oxidation.
2023-02-13 13:00:03
Article from phys.org
Recently, researchers from North Carolina State University revealed a groundbreaking discovery on the oxidation kinetics of multi-cobalt active sites on Co3O4 catalysts for water oxidation.
Using a powerful combination of various spectroscopic and microscopy techniques, the team was able to investigate the precise dynamics of a multi-cobalt active site and detected the consecutive step-by-step oxidation of each cobalt site at the atomic level.
The results of the study offer a more detailed understanding of the water oxidation process. The findings revealed the oxidation of Co3O4 catalyst is significantly faster than the oxidation of single cobalt sites, enabling a greater amount of electrons to be involved in catalysis of the reaction.
The team’s study on the oxidation kinetics of Co3O4 catalysts is the first of its kind and has far reaching implications for the development of improved water oxidation catalysts. The research opens the door for further investigation and innovation in the field of water oxidation catalysis which could help researchers to better design catalysts for future applications in solar energy conversion and artificial photosynthesis.
Overall, the research provides a major breakthrough in the understanding of the oxidation kinetics of Co3O4 catalysts for water oxidation, contributing to our knowledge of this potentially revolutionary technology.


















