A “magic number” of physics may not be as magical as previously thought.
The discovery “came as a big surprise,” says physicist Rituparna Kanungo of Saint Mary’s University in Halifax, Canada, who was not involved in the study. Kanungo explains that there are several advanced theories that attempted to predict and explain the characteristics of oxygen-28, but none of them can account for the observations.
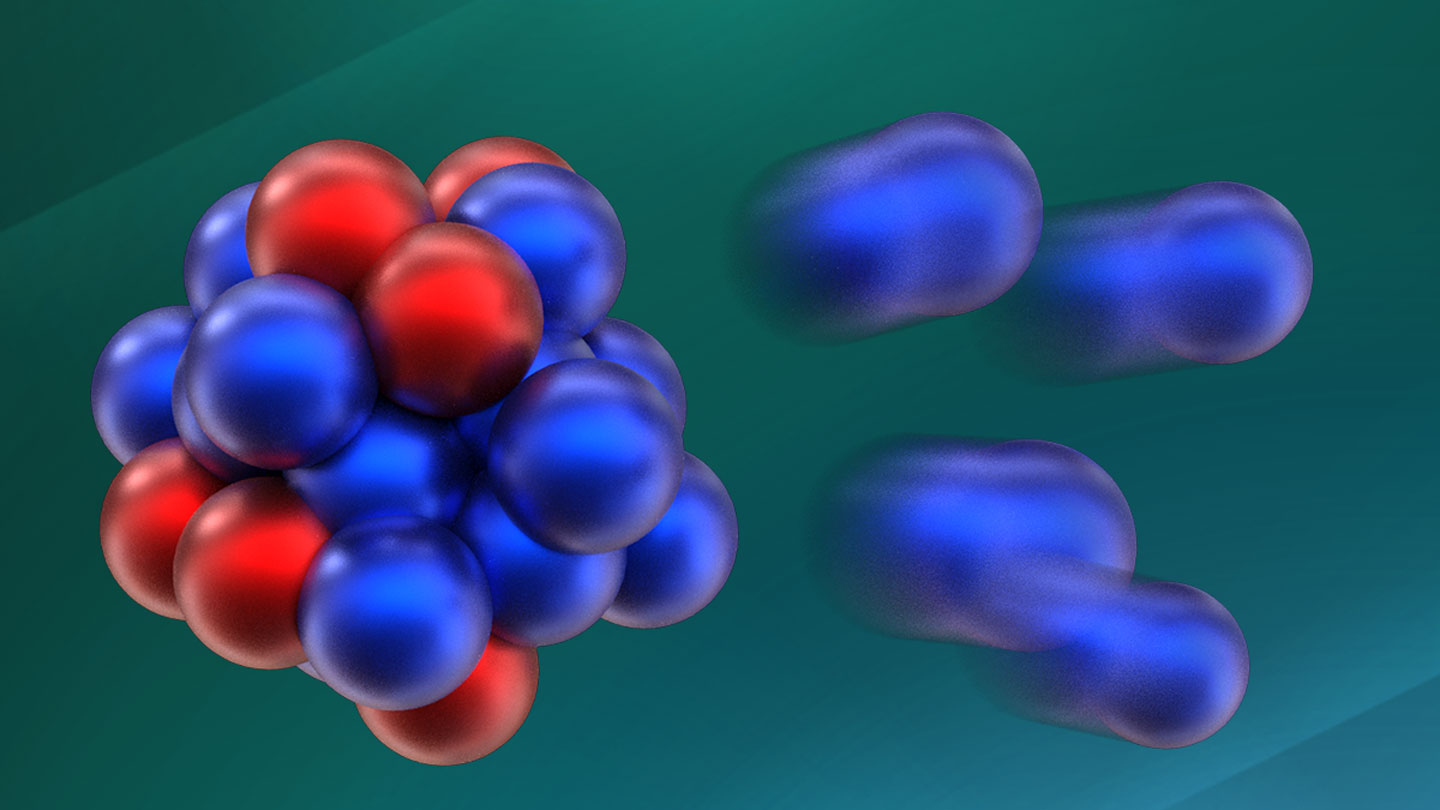
Atomic nuclei consist of protons and neutrons, which are believed to occupy their own distinct “shells” or energy levels that are separated by significant energy gaps. Atomic nuclei with complete outer shells are bound more tightly, resulting in greater stability. These shells fill up when the number of subatomic particles reaches two, eight, 20, 28, 50, 82, and 126 (SN: 10/9/13).
Elements have a fixed number of protons but can have varying numbers of neutrons. For example, the oxygen we breathe contains the isotope oxygen-16, which has eight protons and eight neutrons. This makes it “doubly magic” and exceptionally stable. Oxygen-28, with its 20 neutrons and eight protons, was also expected to be stable due to its doubly magic nature.
2023-09-14 08:00:00
Article from www.sciencenews.org
rnrn