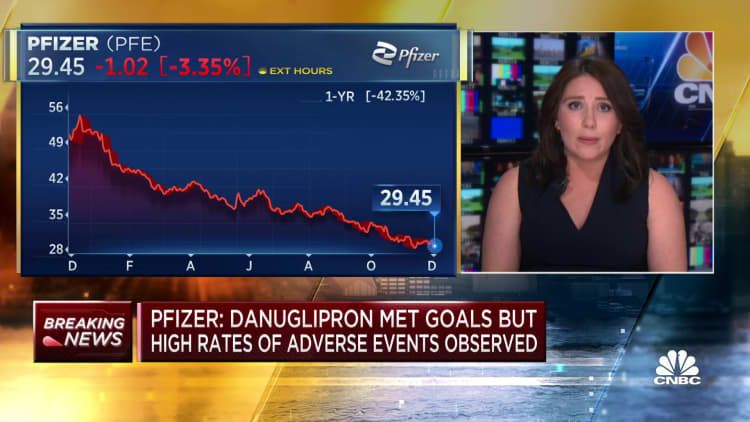
Pfizer announced on Friday that it will no longer continue the development of the twice-daily version of its experimental weight loss pill. This decision comes after obese patients in a mid-stage clinical study experienced significant weight loss but had difficulty tolerating the drug. The study revealed high rates of adverse side effects, mostly mild and gastrointestinal, leading to a significant number of patients discontinuing the drug.
The company stated, “At this time, twice-daily danuglipron formulation will not advance into Phase 3 studies.”
However, Pfizer still plans to release phase two trial data on a once-a-day version of the drug in the first half of 2024. This data will help determine the next steps for the drug. Pfizer will wait for the results before deciding whether to proceed with a phase three study on the once-daily pill, which is considered the more competitive form of the treatment.
Following the announcement of the trial results, Pfizer’s shares fell 4% in premarket trading on Friday.
For more information, read the full article from www.cnbc.com.
2023-12-01 07:58:31
Article from www.cnbc.com
