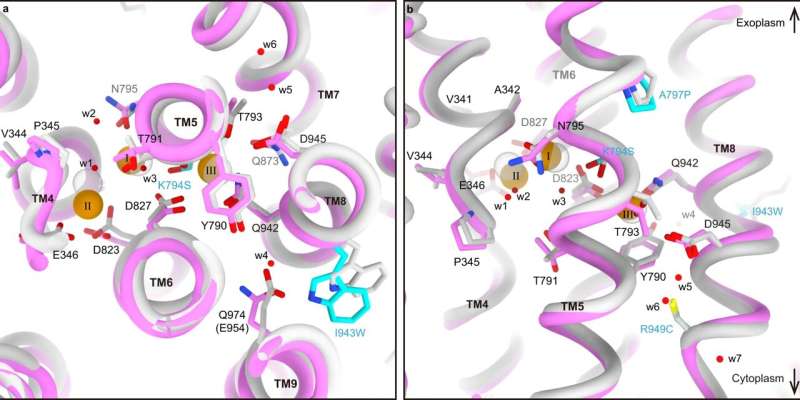
Comparison of the cation-binding web site between SPWC-ngHKA and NKA. a, b Overlapped cation-binding websites of SPWC-ngHKA within the 3Na+·E1-ATP state (pink tubes and sticks) and the (3Na+)E1P-ADP state of NKA (gentle grey, 3wgu) considered from cytoplasmic aspect (a), or parallel to the membrane with the extracellular side-up (b). Mutated residues are indicated with cyan carbons. The three Na+ ions (orange) and 7 water molecules (w1–w7, purple) recognized within the cryo-EM map of SPWC-ngHKA are proven as spheres. All fashions are aligned by their motionless TM7-10 area. Credit: Nature Communications (2022). DOI: 10.1038/s41467-022-32793-0
In a not too long ago printed analysis article, Pablo Artigas, Ph.D., from the Center for Membrane Protein Research on the Texas Tech University Health Sciences Center (TTUHSC) School of Medicine’s Department of Cell Physiology and Molecular Biophysics, and a crew of collaborators utilized purposeful and structural analyses to analyze what structural options of proton/potassium (H+/Okay+) pumps and sodium/potassium (Na+/Okay+) pumps cause them to regulate the passage of salts throughout membrane limitations.
The examine, “Structure and Function of H+/Okay+ Pump Mutants Reveal Na+/Okay+ Pump Mechanisms,” was printed in September by Nature Communications. The analysis crew included Artigas and TTUHSC graduate college students Victoria C. Young, Ph.D., and Dylan J. Meyer, Ph.D.; Hanayo Nakanishi, Ph.D., Atsunori Oshima, Ph.D., and Kazuhiro Abe, Ph.D., from Nagoya (Japan) University; and Tomohiro Nishizawa, Ph.D., from Yokohama (Japan) City University.
In each human cell, the Na+/Okay+ pump transports two potassium (Okay+) ions into the cell and brings out three sodium (Na+) ions. The focus gradients for these ions are wanted for electrical signaling within the mind, coronary heart and muscle, and for the consumption of vitamins and regulation of intracellular concentrations of calcium and protons in all cell sorts. The 4 varieties of Na+/Okay+ pumps localize to totally different tissues. Disease mutations inside three of those Na+/Okay+ pumps trigger neuromuscular, cognitive, endocrine or cardiovascular problems.
Two H+/Okay+ pumps have barely totally different ion-recognition websites and are expressed on the apical aspect of many epithelia, the place they transport one proton (H+) out of the cell and usher in one potassium (Okay+) ion. The gastric H+/Okay+ pump acidifies the abdomen and is the goal of omeprazole, an antacid drug. The non-gastric H+/Okay+ pump participates in Okay+ reabsorption and contributes to acidification of the airway, an necessary a part of cystic fibrosis pathology.
“The two proteins (H+/Okay+ and Na+/Okay+ pumps) are about 70% an identical, so we checked out what minor variations might be accountable for the distinction within the selectivity and within the variety of ions which can be transported,” Artigas defined.
The examine decided that concurrently changing amino acid residues at 4 spots inside the non-gastric H+/Okay+ pump with these current within the Na+/Okay+ pump is sufficient to rework a protein that usually transports one proton for one potassium into one which transports three sodium for 2 potassium, giving new perception into how these proteins choose the ions they need to transport.
“To the perfect of our data, that is the primary demonstration of fixing a protein that completely transports H+ is remodeled right into a protein that completely transports Na+,” Artigas mentioned. “We suppose it might assist others conducting related varieties of work with different membrane proteins to design an analogous change to the selectivity between Na+ and H+. We can do it a method, however we at the moment are attempting to do it the opposite manner: come from the Na+/Okay+ pump to the (non-gastric) H+/Okay+ pump.”
The significance of the non-gastric H+/Okay+ pump within the physique stays largely unknown, however it’s recognized that its inhibition prevents airway infections in an animal mannequin of cystic fibrosis.
“In addition to reworking one kind of protein into one other, due to our structural-biologist collaborators in Japan, we decided the construction of the non-gastric H+/Okay+ pump,” Artigas mentioned. “This construction might be used to develop particular inhibitors to successfully deal with cystic fibrosis sufferers.”
Now that Artigas and his collaborators have efficiently transformed the non-gastric H+/Okay+ pump to a Na+/Okay+ pump, they’re making an attempt a number of different conversions utilizing the gastric H+/Okay+ and Na+/Okay+ pumps.
“We nonetheless have not been in a position to convert the gastric H+/Okay+ pump right into a Na+/Okay+ pump or a Na+/Okay+ pump right into a H+/Okay+ pump to totally perceive the mechanism of selectivity,” Artigas mentioned. “We will use the present and future constructions of the non-gastric H+/Okay+pump to aim producing particular inhibitors to assist deal with cystic fibrosis sufferers.”
Unique underpinnings revealed for abdomen’s acid pump
More info:
Victoria C. Young et al, Structure and performance of H+/Okay+ pump mutants reveal Na+/Okay+ pump mechanisms, Nature Communications (2022). DOI: 10.1038/s41467-022-32793-0
Provided by
Texas Tech University
Citation:
New examine explains mechanisms of salt transport and will assist deal with cystic fibrosis (2022, September 21)
retrieved 21 September 2022
from https://phys.org/information/2022-09-mechanisms-salt-cystic-fibrosis.html
This doc is topic to copyright. Apart from any truthful dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is offered for info functions solely.