The U.S. Food and Drug Administration on Friday approved the country’s first gene-editing treatment, Casgevy, for use in patients with sickle cell disease.
The approval comes about a decade after the discovery of CRISPR technology for editing human DNA, representing a significant scientific advancement. Yet reaching the tens of thousands of people who could benefit from the treatment could be challenging given the potential hurdles — including cost, at $2.2 million per patient — of administering the complex therapy.
Casgevy, co-developed by Vertex Pharmaceuticals and CRISPR Therapeutics, uses Nobel Prize-winning technology CRISPR to edit a person’s genes to treat disease. The treatment was approved by U.K. regulators last month.
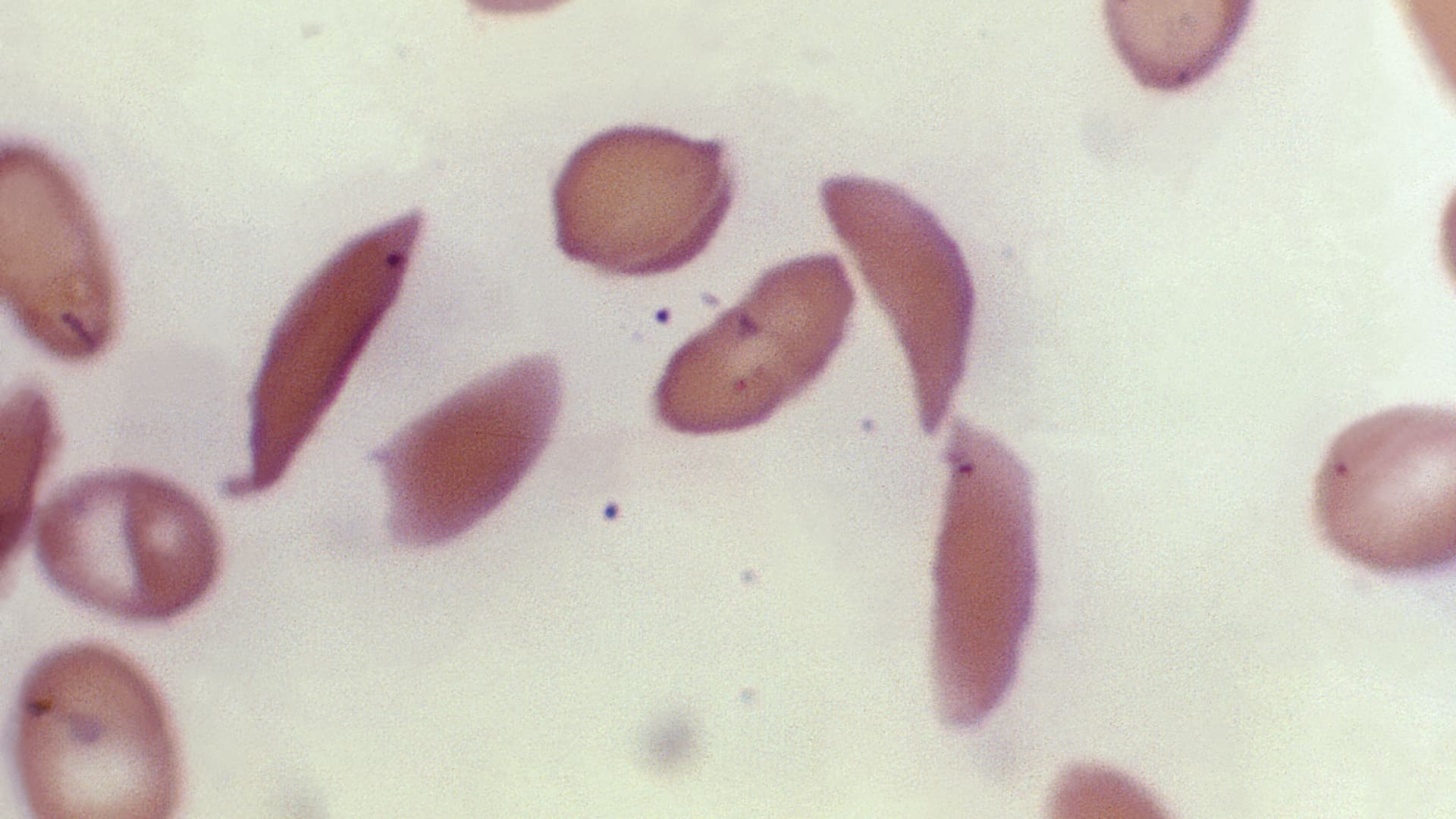
Sickle cell, an inherited blood disorder, causes red blood cells to become misshapen half moons that get stuck inside blood vessels, restricting blood flow and causing what are known as pain crises. About 100,000 Americans are estimated to have the…
2023-12-08 14:25:39
Original from www.cnbc.com
rnrn