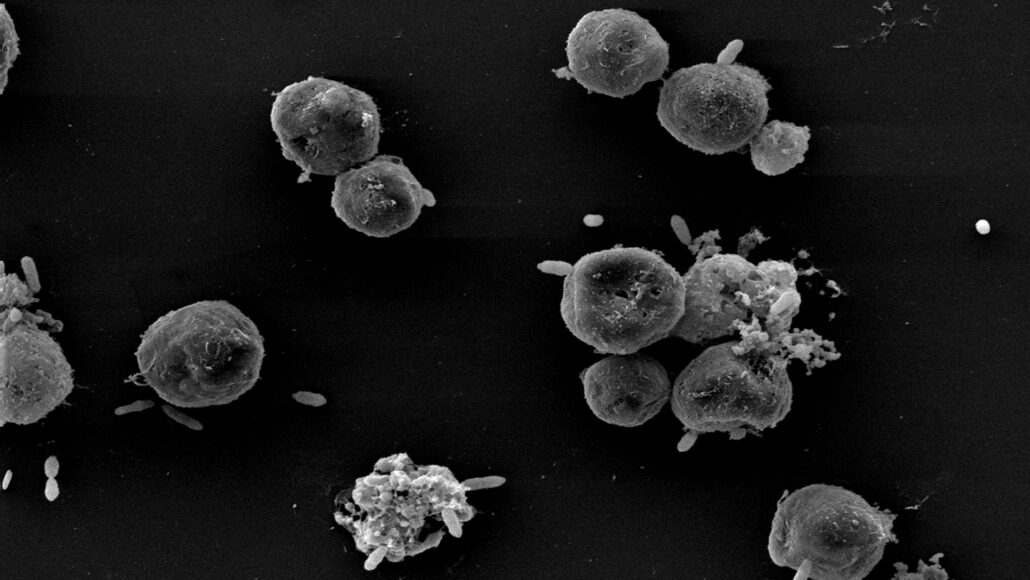
The photosynthesizing plankton Emiliania huxleyi has a dramatic relationship with its bacterial frenemies. These duplicitous bugs help E. huxleyi in exchange for nutrients until it becomes more convenient to murder and eat their hosts. Now, scientists have figured out how these treacherous bacteria decide to turn from friend to foe.
One species of these bacteria appears to keep tabs on health-related chemicals produced by E. huxleyi, researchers report January 24 in eLife. The bacteria maintain their friendly facade until their hosts age and weaken, striking as soon as the vulnerable algae can’t afford to keep bribing them with nutrients. The finding could help explain how massive algal blooms come to an end.
The bacteria is “first establishing what we call the ‘first handshake,’” says marine microbiologist Assaf Vardi of the Weizmann Institute of Science in Rehovot, Israel. “Then it will shift into a pathogen.”
E. huxleyi’s partnership with these bacteria, which belong to a group called Roseobacter, might be best described as a love-hate relationship. The single-celled alga can’t produce the B vitamins it needs on its own, so it offers up nutrients to lure in Roseobacter that can (SN: 7/8/16). The trade is win-win — at least until the bacteria decide they’d be better off slaying and devouring their algal hosts than sticking around in peaceful coexistence.
Sometimes called the “Jekyll-and-Hyde” trait, this kind of bacterial backstabbery shows up everywhere from animal guts to the open seas. But it wasn’t clear before how Roseobacter decide it’s the right moment to murder E. huxleyi.
Vardi’s team exposed a type of Roseobacter that lives with E. huxleyi to chemicals taken from algae that were either young and growing or old and stagnant. The team also introduced the bacteria to extra doses of a certain health-signaling algal chemical.Looking at which genes the bacteria activated in the different experiments revealed how and why they switched from friend to foe.
2023-02-08 08:00:00
Original from www.sciencenews.org
Microbes have been demonstrated to proficiently shift between cooperative and opportunistic behaviour towards their peers. In a new study published in Nature Communications, researchers shed light on the origin of such trends.
In the experiment, conducted by an international research team at the universities of Oxford and CAU Kiel, the scientists explored the relationship between Synechococcus, commonly found in marine environments, and its viral predators, as well as another group of bacteria commonly known as Flavobacteria.
The researchers made the surprising discovery that Flavobacteria bacteria could either act cooperatively, aiding in the defence of Synechococcus against viral attack, or opportunistically, by parasitizing upon the photosynthetic cells and exploiting them for their own gain.
When Synechococcus was surrounded by the collective presence of Flavobacteria, the bacteria were more likely than not to act cooperatively in defending the alga against viral attack. However, when their numbers in the environment were decreased, fewer cooperative bacteria were found, as the opportunistic hosts took over.
This suggests that the density of these bacteria in the environment is a critical determinate in the manner of behaviour they choose to take.
The study found that flavobacteria’s ability to switch between cooperative and opportunistic behaviour is likely down to a simple genetic change – a single-base pair switch in the genes associated with the two different behaviours.
The researchers believe that this finding will help us to understand the base levels of competition between microbes in natural environments, and that it is this competitive behaviour which can lead to the emergence of new species – as well as to certain bacteria becoming more resistant to antibiotics.
Overall, this study highlights the incredible complexity of microbial interactions and once again demonstrates the importance of considering these tiny, but highly influential organisms when trying to gain greater insight into the natural world, and could be an invaluable tool for understanding the dynamics of microbial populations and evolution.
