Better late than by no means, proper? Finally, and after a number of delays, on Tuesday, Novavax (NVAX) introduced it had submitted to the FDA its request for Emergency Use Authorization (EUA) for Covid-19 vaccine NVX-CoV2373.
The setbacks the corporate had encountered on the trail towards the submitting largely revolved round manufacturing points. As such, B. Riley analyst Mayank Mamtani thinks a “key component” in driving approvals will probably be “confidence from government agencies in the company’s global manufacturing infrastructure to deliver on the ensuing demand.”
Until now, Novavax’s strategic companion, the Serum Institute of India (SII), has been answerable for the provision of doses. At the final depend, which incorporates the Indian authorities’s newest export allow of 250 million doses starting this month, the overall tally of doses allotted to be shipped from India has reached over 350 million. The bulk of which has gone towards assembly the dedication to provide the COVAX facility with 1.1 billion doses of the vaccine. It presently stays unclear what the dose allocation plan is for upper-income nations.
However, Mamtani notes that SII’s manufacturing capability, together with SK Biosciences’ “almost comparable” progress on scale-up manufacturing, signifies Novavax is “gaining substantial supply to meet APA commitments to upper income countries, via CDMO arrangements with these two strategic partners.” All this whereas the corporate ploughs forward with CMC knowledge era actions in 10+ North America and EU services.
Mamtani additionally thinks that as a result of current vital “COVID disease burden” coupled with the “unequivocal real-world evidence of diminished antibody-mediated vaccine efficacy,” the broadly held view that omicron has lowered the extent of urgency is misplaced. Accordingly, with “imminent” U.Ok. MHRA approval, adopted by the doable US EUA, Novavax is well-positioned to offer the “only alternative to mRNA vaccines in order to ensure sufficient immunization level exists in the population prior to the emergence of next COVID variant & ensuing wave of cases.”
Story continues
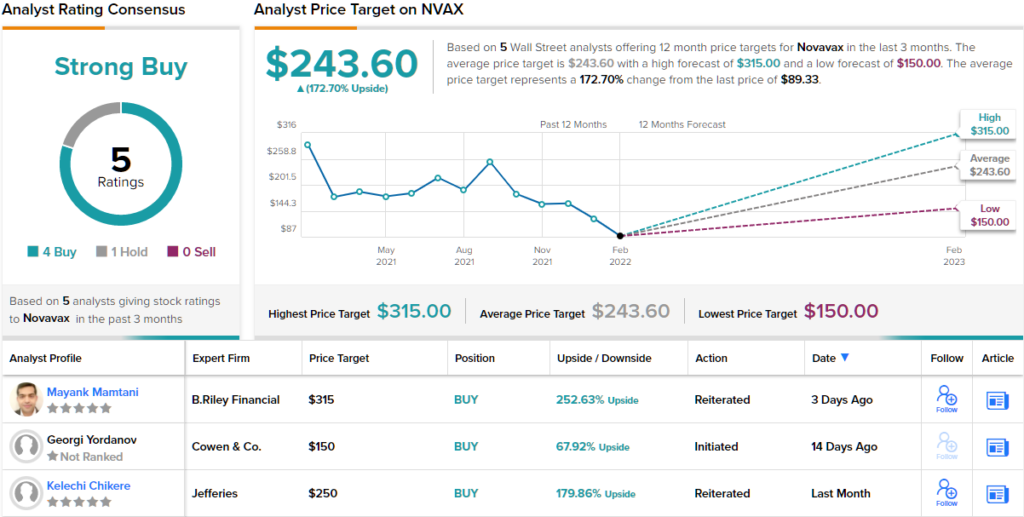
As such, Mamtani implores buyers reap the benefits of an “attractive additional entry point,” to scoop up NVAX shares on a budget. The analyst’s Buy ranking is backed by a $315 value goal, suggesting shares will rise by 231% within the 12 months forward. (To watch Mamtani’s monitor file, click on right here)
Overall, this vaccine maker will get a Strong Buy ranking from the consensus of Wall Street’s analysts; the 5 current evaluations on the inventory embrace 4 Buys and 1 Hold. Novavax shares are promoting for $89.33, and the common value goal of $243.60 suggests room for ~172% progress within the 12 months forward. (See Novavax inventory forecast on TipRanks)
To discover good concepts for shares buying and selling at enticing valuations, go to TipRanks’ Best Stocks to Buy, a newly launched instrument that unites all of TipRanks’ fairness insights.
Disclaimer: The opinions expressed on this article are solely these of the featured analyst. The content material is meant for use for informational functions solely. It is essential to do your personal evaluation earlier than making any funding.