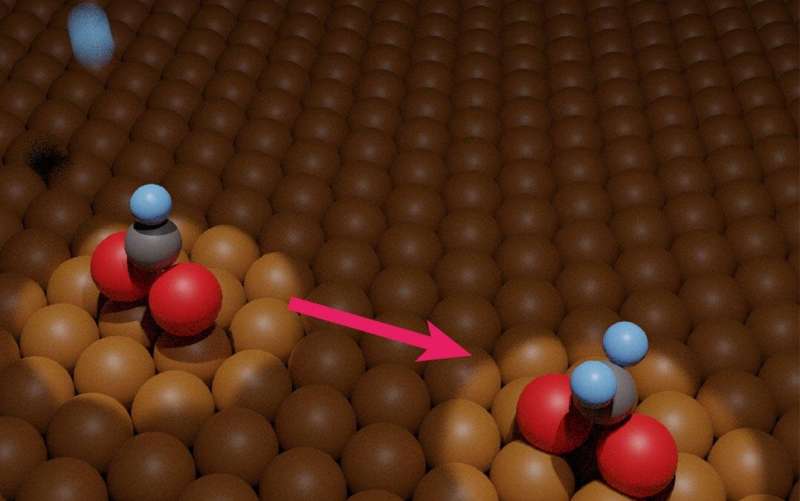
Researchers from the University of Tsukuba and collaborating companions experimentally measured hydrogenation of metal-adsorbed formate. This growth will facilitate sensible conversion of carbon dioxide pollutant into methanol gasoline. Credit: University of Tsukuba
Carbon dioxide air pollution continues to alter the worldwide local weather. Researchers know find out how to pinpoint such air pollution, even on a regional and near-real-time foundation. As a part of an answer to carbon dioxide air pollution, many research deal with find out how to convert this pollutant right into a gasoline, akin to methanol. Copper-based catalysts are a instrument for such conversions. Understanding the corresponding step-by-step chemistry is crucial for optimizing conversion of carbon dioxide pollutant into methanol gasoline. However, the main points of this chemistry stay unclear; experiments are wanted to check hypotheses which might be at present based mostly on laptop simulations.
Now, in a research lately revealed in Journal of the American Chemical Society researchers from the University of Tsukuba and collaborating companions have experimentally measured hydrogenation of copper-adsorbed formate. This research will assist researchers optimize vital steps within the aforementioned pollutant-to-fuel course of, and thus speed up methanol manufacturing.
“Hydrogenation of carbon dioxide into methanol is a possible key expertise for producing gasoline and chemical feedstocks, however optimizing the response stays troublesome,” explains Dr. Kotaro Takeyasu, senior creator. “That’s as a result of it is troublesome to experimentally detect chemical intermediates within the step-by-step response mechanism.”
Infrared reflection absorption spectroscopy and temperature-programmed desorption have been vital to acquiring two foremost findings. First, at a temperature of 200 Kelvin, publicity to atomic hydrogen corresponded to hydrogenation of adsorbed formate. The actual chemical nature of the product is not but clear. It was additionally discovered that, at a temperature of 250 Kelvin, the hydrogenated formate decomposed again into adsorbed formate or gaseous formaldehyde, in a 96:4 ratio.
“On the premise of our experimental and computational work, the activation vitality of the hydrogenation of adsorbed formate is roughly 121 kilojoules per mole,” states Dr. Takeyasu. “Our outcomes are according to reported outcomes of methanol synthesis research.”
Copper-zinc alloys are notably widespread on this line of labor. The analysis group is at present investigating how the activation energies reported within the current research evaluate with notably helpful catalytic alloys, which additionally require experimental and computational investigations.
The outcomes of this research will assist researchers optimize methanol manufacturing from carbon dioxide. Such work will assist convert an atmospheric pollutant into gasoline for autos, and chemical feedstocks for trade. It supplies a way of including worth to carbon dioxide, which is often thought-about to be waste. By optimizing the hydrogenation response described right here, researchers may need a brand new instrument for making most use of restricted sources.
Discovery of a brand new catalyst for extremely energetic and selective carbon dioxide hydrogenation to methanol
More info:
Kotaro Takeyasu et al, Hydrogenation of Formate Species Using Atomic Hydrogen on a Cu(111) Model Catalyst, Journal of the American Chemical Society (2022). DOI: 10.1021/jacs.2c02797
Provided by
University of Tsukuba
Citation:
Caught within the act: Key chemical intermediates in pollutant-to-fuel response recognized (2022, July 5)
retrieved 5 July 2022
from https://phys.org/information/2022-07-caught-key-chemical-intermediates-pollutant-to-fuel.html
This doc is topic to copyright. Apart from any truthful dealing for the aim of personal research or analysis, no
half could also be reproduced with out the written permission. The content material is offered for info functions solely.