Maybe you’ve heard that the pandemic is over within the United States. (It’s not.) Masks are not required in most locations and enormous gatherings have gotten commonplace once more. Most of the nation is within the inexperienced, in response to the U.S. Centers for Disease Control and Prevention’s monitoring web site, which displays COVID-19 transmission and hospitalization charges. Many individuals have been counting on vaccines to manage outbreaks, and there may be renewed consideration on getting newly obtainable remedies to sick individuals.
But COVID-19 coronavirus instances are on the rise once more with greater than 1 / 4 of counties reporting excessive ranges of transmission. And these are official numbers. No one actually is aware of what number of at-home checks come again optimistic and are by no means reported (SN: 4/22/22). Those instances are driving hospitalization charges up, with pockets of yellow and orange popping up on the CDC’s map, indicating that hospitals are coming into the hazard zone for being overwhelmed. Deaths have remained pretty low. That might change if one other wave of an infection sweeps the nation.
“Maybe we think we’re on the edge of the woods, but we’re not out of it yet,” says Mark Denison, a COVID-19 coronavirus researcher at Vanderbilt University Medical Center in Nashville.
Sign up for e-mail updates on the newest COVID-19 coronavirus information and analysis
Preventing hospitalizations and deaths is what vaccines have been designed to do, however even the excellent COVID-19 vaccines aren’t good. And with new immune-evasive variants of the COVID-19 coronavirus, even vaccinated individuals and those that beforehand had COVID-19 — notably the aged or individuals with weakened immune programs or different well being issues — can wind up within the hospital.
The subsequent line of protection in opposition to that unhealthy final result are three antiviral medication and a monoclonal antibody that will preserve individuals newly identified with COVID-19 from changing into severely ailing and dying.
The test-to-treat initiative
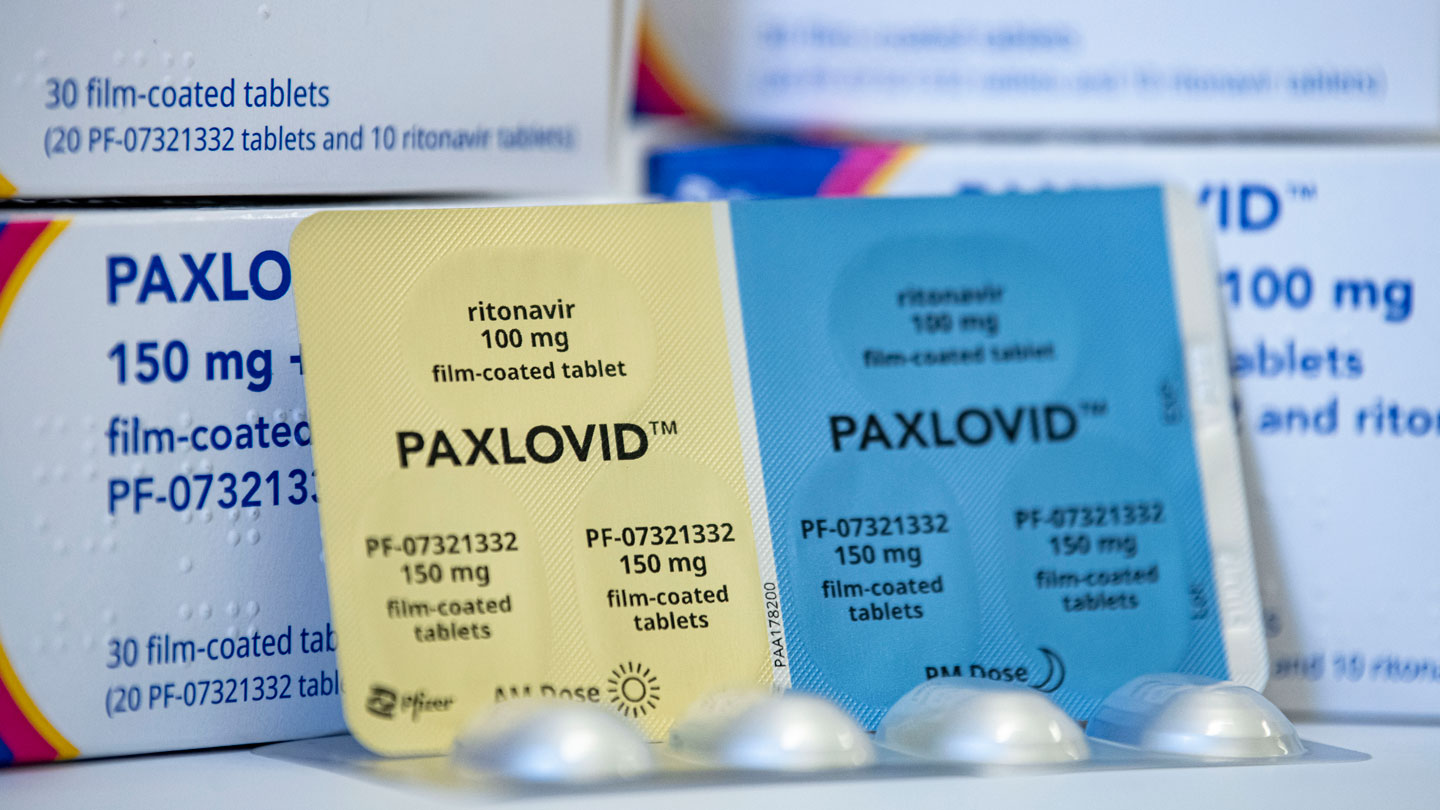
About 20,000 pharmacies, hospitals, pressing care facilities and different locations have the antiviral tablets Paxlovid (a combo of two medicines), made by Pfizer. The federal authorities plans to increase that quantity to 40,000 within the coming weeks, Ashish Jha, the White House COVID Response director, stated April 26 throughout a information briefing. Many of these websites even have molnupiravir (Logevrio) antiviral tablets, made by Merck (SN:12/2/21). The growth is a part of the federal authorities’s test-to-treat initiative to make testing and therapy broadly obtainable.
In far more restricted provide are intravenous doses of monoclonal antibodies and the antiviral drug remdesivir — the one antiviral drug absolutely authorised by the U.S. Food and Drug Administration for treating COVID-19.
One preventative therapy is a monoclonal antibody known as Evusheld. It is for individuals with weakened immune programs who could not reply to vaccines or who can’t be vaccinated. In a scientific trial, it decreased the danger of growing symptomatic COVID-19 infections by 82 p.c in contrast with a placebo, researchers reported April 20 within the New England Journal of Medicine. That therapy is plentiful however isn’t getting used a lot.
Remdesivir, or Veklury as its maker Gilead Sciences calls it, was authorised by the FDA in 2020 for treating hospitalized sufferers (SN:4/29/20). A current scientific trial confirmed that the drug decreased the possibility of hospitalization by 87 p.c in nonhospitalized individuals at excessive danger of extreme illness, researchers reported within the January 27 New England Journal of Medicine. Such optimistic outcomes prompted the FDA to increase its approval to outpatient use in individuals 12 and older. Then on April 25, the FDA approved the drug for use in kids 12 and youthful, making it the primary antiviral COVID-19 therapy obtainable for younger children.
The principal draw back to remdesivir is that it needs to be administered intravenously. “There’s nothing convenient about that,” says Abraar Karan, an infectious illnesses doctor at Stanford University. Researchers are engaged on an oral type of remdesivir. Early experiments counsel it’s efficient at defending mice in opposition to extreme sickness. Tests nonetheless need to be achieved to see if it really works for individuals, too.
Two different medication, Paxlovid and molnupiravir, are available tablet kind. They are theoretically extra available, however many individuals don’t find out about them or get them. And some social media posts counsel that it’s not as simple because it sounds for everybody to put their fingers on the medication after a optimistic COVID-19 take a look at, even after they do find out about them.
“It’s not as simple as, ‘We have a pill, now everything is solved,’” Karan says.
That’s partly as a result of some docs aren’t conscious that they will or ought to prescribe the medication for his or her high-risk sufferers who get contaminated with the COVID-19 coronavirus, says Jason Gallagher, a pharmacist and infectious illnesses physician at Temple University in Philadelphia. “There’s a lot of education that needs to occur on the medical side, also,” he says.
Sign Up For the Latest from Science News
Headlines and summaries of the newest Science News articles, delivered to your inbox
Thank you for signing up!
There was an issue signing you up.
Ideally, individuals would speak to their docs about whether or not they’re candidates for the medication earlier than they get contaminated, Karan says. And individuals who don’t have a health care provider have to know their choices, as a result of timing is vital: The tablets must be began inside three to 5 days of signs showing.
Access to the medication is getting higher, but it surely’s nonetheless patchy. In the curiosity of being ready, I went to COVID.gov to study the place I might get the medication in case I catch the COVID-19 coronavirus. I’m comparatively fortunate. There are seven test-to-treat websites inside 5 miles of my home simply outdoors of Washington, D.C., although one is a navy hospital not open to most of the people. And I spoke to my physician to ask if I ought to take Paxlovid if I get COVID-19. He stated I ought to and that if I do a house take a look at, I can name with the end result, and he’ll name a prescription in to my pharmacy.
My mother in rural Nebraska is in a distinct state of affairs. She must drive greater than 25 miles to the closest test-to-treat web site. Many different rural areas have pharmacies that may fill prescriptions, however individuals must go elsewhere to be examined and get a health care provider to prescribe the remedy. For occasion, residents of Gypsum, Colo., must get examined someplace, get a prescription from a health care provider, drive about six miles to get the prescription crammed, or journey practically 100 miles to the Denver suburbs for a test-to-treat web site.
Drawbacks to those medication
There are different wrinkles moreover entry to utilizing the medication. “For all of the therapeutics, they have been studied primarily in unvaccinated people who are at risk of going on to develop severe disease,” says Gallagher. “So we basically end up projecting that data onto the population we have now, which is largely vaccinated.” It’s not clear that each one that has well being situations that put them in danger wants the medication, particularly if they’ve gotten a vaccine booster shot. The medication would possibly lower signs, however there’s no actual proof to point out they’re efficient in making individuals really feel higher quicker, he says.
The medication are good at retaining individuals out of the hospital, although. Paxlovid decreased the relative danger of hospitalization and dying by about 89 p.c, researchers reported within the April 14 New England Journal of Medicine. Its uncomfortable side effects included a distorted sense of style, and a small variety of individuals developed diarrhea.
See all our protection of the COVID-19 coronavirus outbreak
A much bigger disadvantage is among the medication within the Paxlovid combo. Paxlovid’s principal drug, nirmatrelvir, works by inhibiting an enzyme that the virus wants to duplicate. It is taken with a drug known as ritonavir, which retains nirmatrelvir from being damaged down by enzymes within the physique. Ritonavir can react with different medicines, like cholesterol-lowering statins, making ranges of these medicines within the physique dangerously excessive. There are methods to work round that, although, Gallagher says. For occasion, cholesterol-lowering medicines might be halted for a short while. “I can’t think of a single person who can’t stop taking those for a week or two” whereas being handled for COVID-19, he says.
Another wrinkle is relapse. One preprint and different anecdotal accounts have reported that some individuals have relapses of the virus a couple of days after stopping Paxlovid. No one actually is aware of how frequent that’s or why it occurs. The U.S. National Institutes of Health are collaborating with the CDC and the FDA to raised perceive the phenomenon, in response to an announcement the National Institute of Allergy and Infectious Diseases despatched to me. But the companies haven’t any formal research underway.
Experts I talked to had some concepts about what may be occurring. It might be that the immune system “chills out” whereas persons are taking the drug, giving the virus the chance to rebound as soon as the drug is stopped, Gallagher speculates. Or the virus would possibly conceal in sure locations within the physique that the drug has bother reaching, Denison says. Both of these conditions may be solved by giving individuals the drug for longer, they are saying.
But the FDA rejected that suggestion in a put up citing John Farley, director of the company’s Office of Infectious Diseases. Similar relapses occurred to individuals taking placebo tablets in Pfizer’s scientific trial of the drug, so the viral rebound is probably not associated to taking the drug, Farley harassed. And there was no indication that individuals get sicker and are hospitalized after a relapse. There’s additionally no information to say whether or not individuals would profit from taking Paxlovid longer or getting one other spherical of the tablets if they’ve a relapse, he stated.
Resistance to the drug is one more reason infections would possibly relapse. “I think drug resistance is the least likely explanation,” says Denison. For resistance to develop, the virus has to duplicate, which it may’t do when the drug is round. Still, researchers ought to look ahead to mutations in viruses taken from relapsed sufferers that may point out resistance, he says.
None of the medication are more likely to stop waves of an infection. For occasion, Paxlovid barely decreased the possibility of contracting COVID-19 in individuals who have been uncovered to contaminated family members, however the end result wasn’t statistically significant, Pfizer reported April 29 in a information launch. That means it’s not nice as a preventative and gained’t have an effect on case counts.
“What [the drugs] could flatten is a surge in hospitalizations and deaths,” Gallagher says. But, he notes, individuals at excessive danger of extreme illness shouldn’t depend on the medication alone to save lots of them. “I don’t think that any of these therapeutics is as good as a booster.”
Trustworthy journalism comes at a value.
Scientists and journalists share a core perception in questioning, observing and verifying to achieve the reality. Science News experiences on essential analysis and discovery throughout science disciplines. We want your monetary assist to make it occur – each contribution makes a distinction.
Subscribe or Donate Now