Delocalized electron precursor generated by multiphoton excitation at 400 nm. Credit: Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2216480120
Hydrated electrons play a major role in many physical, chemical and biological processes. They are not bound to an atom or molecule and are free in the solution. Since they are only ever created as an intermediate product, they are extremely short-lived.
The team from the Cluster of Excellence Ruhr Explores Solvation RESOLV at Ruhr University Bochum was able to observe for the first time in a novel experiment how the hydrated electron affects the solution during its lifetime. The researchers led by Professor Martina Havenith-Newen report in the journal Proceedings of the National Academy of Science.
“As the simplest anion, hydrated electrons represent a model system that is relevant in a multitude of radical chemical processes,” says Martina Havenith-Newen. “For example, it plays an important role in energy transfer during photo- and electrochemical phenomena, in atmospheric chemistry, in radiation damage of biological substances and in medical therapy.” This has earned the hydrated electron the ongoing attention of experimental and theoretical groups for several decades.
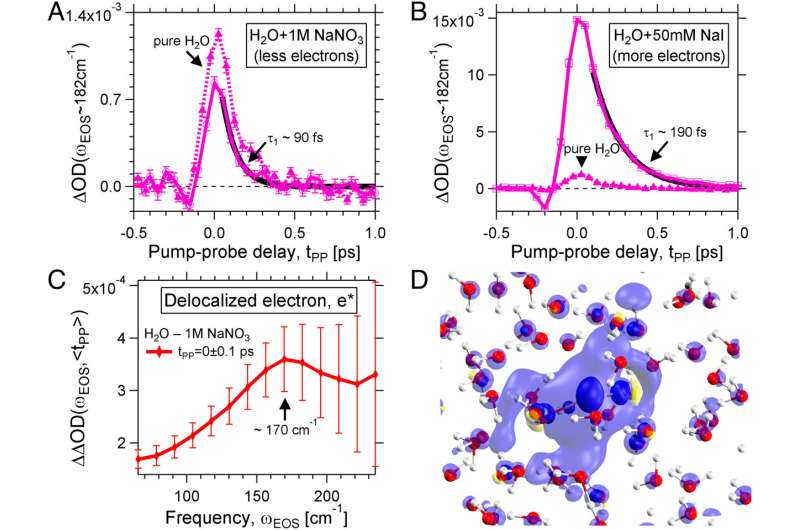
RESOLV researchers have set up a novel experiment to follow the formation and temporal evolution of the hydrated electron from the perspective of the solvent: “Immediately after its generation by means of an intense laser beam, we were able to observe a delocalized electron,” Martina Havenith-Newen says.
The charge distribution extends over 20 angstroms. Within 500 femtoseconds, the charge is localized and a surprisingly stable localized electron emerges, whose fingerprint in the water network the researchers were able to observe for the first time due to the sensitivity of the experiment in the terahertz range.
“In addition, we could observe a water quake or a tsunami,” says Martina Havenith-Newen. The team was able to demonstrate that this phenomenon is caused by the sudden charge separation during the formation of the hydrated electron. In contrast to atomic, negatively charged ions, the water network in the immediate vicinity is looser and not more stable. This means that the individual water molecules in the immediate vicinity of the electron can move more freely than in the water.
2023-02-18 08:00:03
Link from phys.org
We often hear about the destruction of natural disasters and the ravaging damage that they can cause to cities and towns. One of the most destructive and volatile of these types of events is the tsunami, a large wave caused by a violent seismic disturbance in the ocean that can spread to cause destruction both on land and at sea.
Recently, a group of scientists from Australia observed that, even on a small and controlled scale, tsunamis can cause drastic changes to the environment. This was done by assessing the effects that a tsunami wave produces in a glass of water.
To begin, the team set up a 20 cm tall glass container which was filled with water. Above the water, they then laid out a 1.2 m long sheet of rubber where they were able to simulate a tsunami wave with the aid of compressed air. As the lung waves were pushed out, they propagated down the water in the container and traveled back and forth across the width of the water.
The experimental results were rather surprising. When the waves traveled down the container, they created an interesting type of wake due to their breakage of the surface tension of the water. This caused changes in the capillary pressure, which caused a wide array of inter-plate environmental and chemical effects to form. These included changes to chemical concentrations, temperature, salinity, and even the direction of the waves to some degree.
Despite the relatively small scale of the experiment, it did provide us with some useful insight into the effects of tsunamis on the environment. It shows us just how large and expansive the effects of these large waves of water can be. In understanding them on a smaller scale in this experiment, it also helps us to better visualize the magnitude of these effects. This knowledge gives us an invaluable insight into the importance of protecting ourselves from them by better understanding the effects they can have and how to prepare ourselves to brace them if they were to occur.
