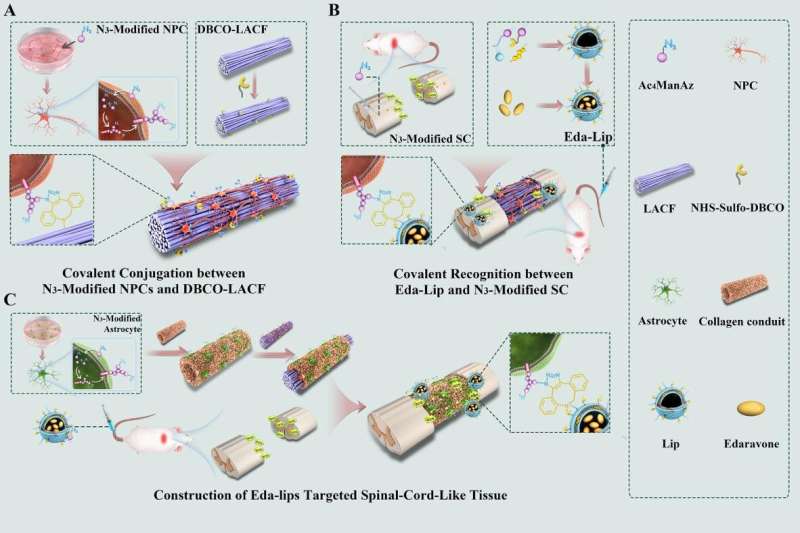
Schematic illustration of engineered spinal cord tissues via covalent interactions between cell and biomaterials. Credit: IGDB
In a recent study published in Science Advances, a research team led by Profs. Dai Jianwu and Zhao Yannan at the Institute of Genetics and Developmental Biology (IGDB) of the Chinese Academy of Sciences demonstrated a strategy for covalent conjugation between biomaterials and cells to construct spinal cord-like tissue with drug-guiding function for spinal cord injury (SCI) repair.
SCI repair is one of the world’s most challenging medical problems. Engineered spinal cord-like implants based on stem cells and biomaterials provide a new method for improving therapeutic outcomes for SCI. The adhesion of stem cells to scaffolds can be regulated by the stiffness, surface topological structure and porosity of the scaffold, as well as by the modification of integrin ligand or adhesion molecules based on noncovalent interactions.
However, the dynamic reversibility of noncovalent adhesion may be adverse for cell retention in complicated environments after SCI.
Unlike noncovalent interactions, covalent interactions form stronger bonds that hold atoms together by sharing electrons. However, whether covalent conjugation between biomaterials and stem cells can be used for tissue engineering has remained elusive.
In this study, the researchers first metabolically labeled human neural progenitor cells (NPCs) with Ac4ManNAz and modified the collagen scaffold NeuroRegen with dibenzocyclooctyne (DBCO) groups to create covalent interaction between biomaterials and cells.
Covalent conjugation significantly prolonged cell retention on the scaffolds, induced cells to spread along the direction of fibers and promoted cell differentiation, according to the researchers.
2023-02-15 07:00:03
Article from phys.org
Recent decades have seen amazing advancements in the field of engineering, specifically in the area of tissue engineering. A fundamental goal of tissue engineering is the creation of artificial, drug-guided tissue that mimics authentic spinal cord-like tissue. This tissue has the potential to repair spinal cord injuries, a life-altering condition which affects millions worldwide.
In order to create this tissue, scientists use a process known as bioprinting. This process involves exposing live cells to engineered materials in order to create a custom tissue. This engineered spinal cord-like tissue is specifically designed to aid in spinal cord repair. The live cells then develop into tissue that forms the physical nature of the spinal cord fragments.
Once developed, the tissue is exposed to certain drugs that help to guide nerve signaling along the engineered spinal cord-like tissue. Drugs that are used include neurotropic and neurogenic drugs, which help to encourage nerve growth and reparation. The drug-guided process is used in conjunction with electrical impulses which aid in stimulating axonal regrowth.
In recent clinical trials, scientists have been successful in using engineered tissue for the repair of a number of spinal cord injuries. The spinal cord injuries repaired by this drug-guided tissue have seen tremendous results, with functional movement and sensation being regained in some cases.
The advancements in tissue engineering have been groundbreaking and truly revolutionary. On top of the potential for spinal cord repair, it also opens the possibility to create tissue for other medical conditions, such as Parkinson’s disease and stroke.
This drug-guided, engineered tissue has the potential to revolutionize the field of medicine, both in the treatment of spinal cord injuries and the prevention of many other medical afflictions. Research into this exciting field of tissue engineering is ongoing and will bring a brand new realm of advancement.
