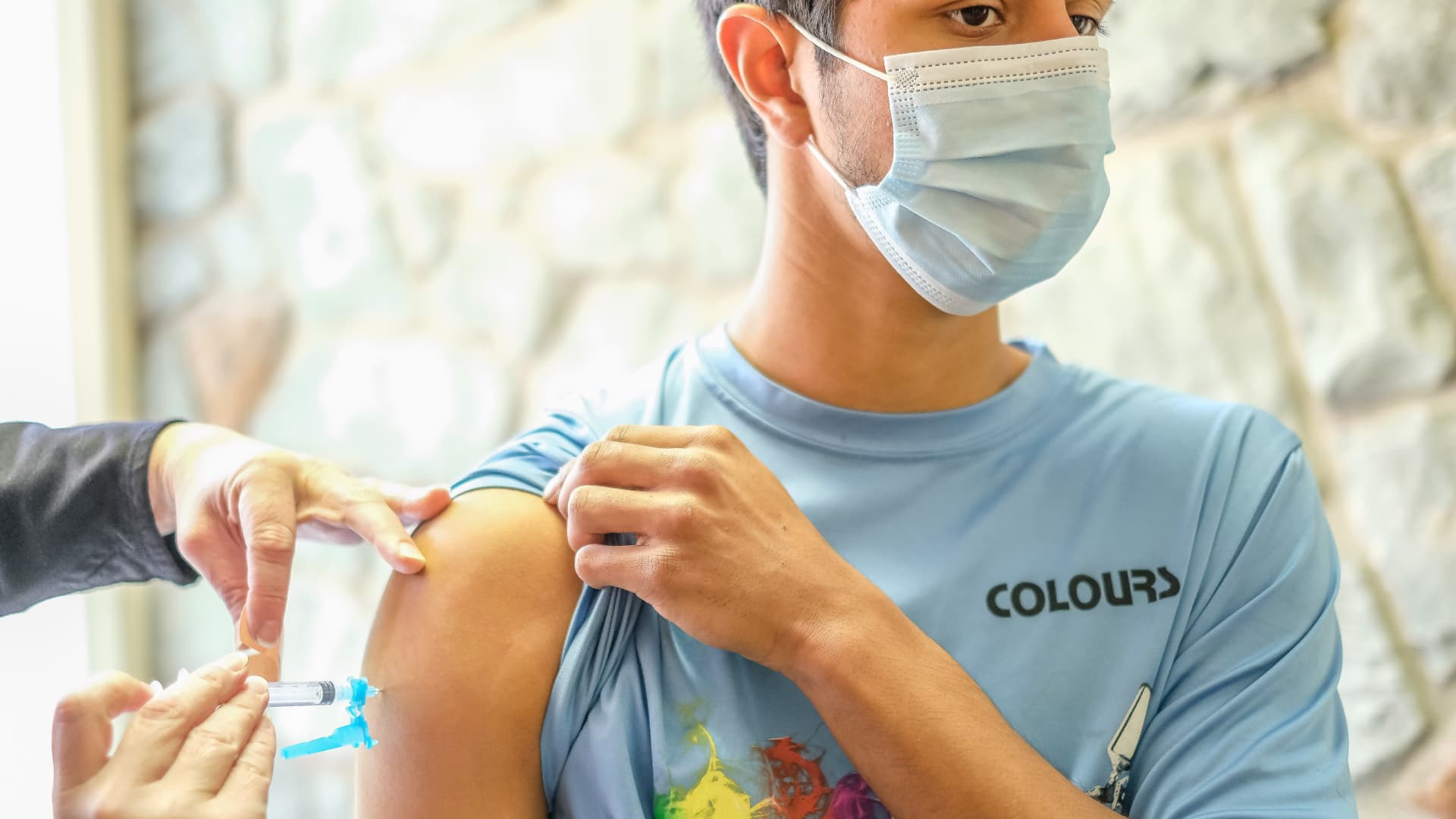
The Centers for Disease Control and Prevention is anticipated to clear Moderna’s two-dose Covid-19 vaccine for kindergartners via excessive schoolers for public distribution this week after the company’s panel of unbiased vaccine consultants unanimously voted Thursday to advocate the pictures.
The committee endorsed Moderna’s vaccine for youths ages 6 to 17 after inspecting its security and effectiveness throughout a public assembly. CDC Director Dr. Rochelle Walensky is anticipated to log out on the advice later Thursday, the ultimate step earlier than pharmacies and physician’s places of work can begin administering the pictures.
The CDC endorsed Moderna’s vaccines for infants via preschoolers, ages six months to 5-years-old, on Saturday. Vaccinations began this week for that age group.
Moderna’s pictures for older children will not have a direct affect on the U.S. vaccination marketing campaign, apart from offering mother and father with an alternative choice to select from. Previously, solely Pfizer’s vaccine was approved for kindergartners via excessive schoolers, although uptake has been lackluster. Two-thirds of children ages 5 to 11 and 30% of adolescents ages 12 to 17 have not been vaccinated in opposition to Covid but.
More than 600 children in these age teams have died from Covid through the pandemic and greater than 45,000 have been hospitalized, based on the CDC. Nearly 11 million children ages 5 to 17 have caught Covid through the pandemic.
Kids ages 6 to 11 obtain smaller 50 microgram Moderna pictures, whereas adolescents ages 12 to 17 would obtain the identical dosage as adults at 100 micrograms.
Moderna initially requested the Food and Drug Administration to authorize its vaccine for adolescents ages 12 to 17 greater than a yr in the past, however the regulator held off after different nations raised concern the corporate’s pictures is perhaps related to the next threat of coronary heart irritation, or myocarditis, than Pfizer’s vaccine.
There are not any head-to-head comparisons within the U.S. of coronary heart irritation in children who get Pfizer’s or Moderna’s pictures as a result of Moderna’s vaccine was solely approved for adults till this month. However, comparisons between Pfizer and Moderna pictures in younger adults seems to indicate that the speed of myocarditis is barely increased in Moderna recipients, although information shouldn’t be constant throughout the assorted U.S. surveillance programs.
“Some proof means that myocarditis and pericarditis dangers could also be increased after Moderna than after Pfizer. However, the findings will not be constant in all US monitoring programs,” Dr. Tom Shimabukuro, an official on the CDC vaccine security unit, informed the committee.
The out there U.S. information on myocarditis amongst children ages 6 to 17 is predicated on unintended effects reported from Pfizer’s vaccine as a result of Moderna’s pictures hadn’t been approved for this age group but. Pfizer and Moderna’s pictures use related messenger RNA know-how.
The CDC has recognized 635 circumstances of myocarditis amongst youngsters ages 5 to 17 after vaccination out of 54 million Pfizer doses administered. The threat of myocarditis after Pfizer vaccination is highest after the second shot amongst boys ages 12 to 17. Myocarditis is barely elevated amongst boys ages 5 to 11 after the second dose of Pfizer’s vaccine, although it’s a lot decrease than adolescents.
Boys ages 16 to 17 reported 75 myocarditis circumstances per 1 million second Pfizer doses administered whereas boys ages 12 to fifteen reported about 46 myocarditis circumstances, based on CDC information. Boys ages 5 to 11 reported 2.6 myocarditis circumstances per million second Pfizer doses administered.
People who’ve develop myocarditis after vaccination are usually hospitalized for just a few days as a precaution earlier than being despatched house. Most sufferers made a full restoration 90 days after their prognosis, based on a CDC survey of health-care suppliers.
The CDC has discovered that the chance of myocarditis is increased from Covid an infection than vaccination. Myocarditis in youngsters is often attributable to viral infections.
Dr. Sara Oliver, a CDC official, stated the chance of myocarditis after Moderna vaccination in youngsters and adolescents is unknown, although information from adults suggests the chance may very well be increased than Pfizer’s pictures. However, Oliver stated extending the interval between the primary and second dose to eight weeks might decrease the chance of myocarditis primarily based on information shared by well being officers in Canada.
The most typical unintended effects amongst children ages 6 to 17 throughout Moderna’s medical trials had been ache on the injection web site, fatigue, headache, chills, muscle ache and nausea. There had been no confirmed circumstances of myocarditis through the trials.
It’s unclear how efficient the pictures shall be in opposition to the omicron variant. The medical trials had been carried out in periods when different Covid strains had been dominant. The pictures for adolescents ages 12 to 17 had been about 90% efficient at stopping sickness from the unique Covid pressure and the alpha variant, whereas the pictures for youths ages 6 to 11 had been greater than 76% efficient at stopping sickness from the delta variant, based on the Food and Drug Administration’s overview of medical trial information.
However, the Covid vaccines have hassle combating the omicron variant, which is now dominant, as a result of it has so many mutations. Third pictures have considerably elevated safety in different age teams. Moderna is finding out booster pictures for youths that concentrate on omicron with information anticipated later this summer time.
“We would count on to be addressing this hole in booster dose suggestions over the summer time and into early fall,” stated Dr. Doran Fink, a senior official on the FDA’s vaccine division.