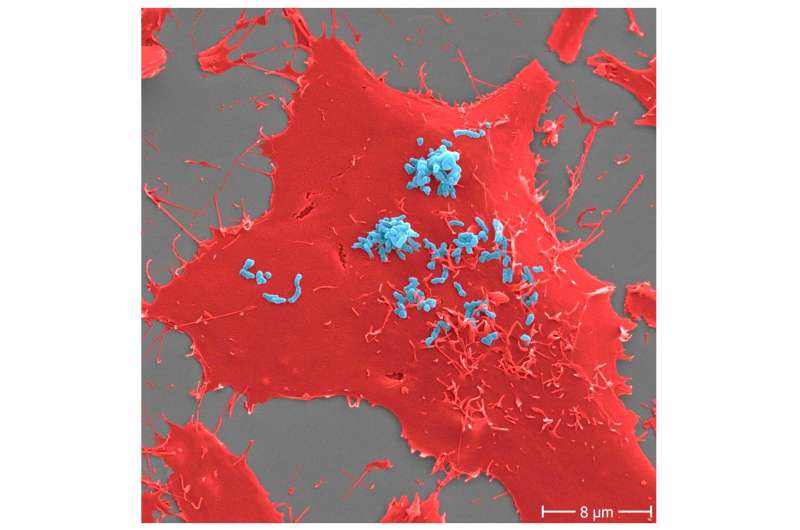
Adhesion of Bartonella henselae to human cells. B. henselae (pressure Marseille) micro organism (mild blue) in an early stage an infection course of (30 min) to human HeLa-229 cells (pink). Adhesion to host cells is mediated by particular interactions between B. henselae floor proteins and parts of the host extracellular matrix together with molecules similar to fibronectin or collagen. Scale bar: 8 μm. Credit: Microbiology Spectrum (2022). DOI: 10.1128/spectrum.00598-22
Researchers from University Hospital Frankfurt and Goethe University Frankfurt have unraveled how micro organism adhere to host cells and thus taken step one in direction of creating a brand new class of antibiotics.
The adhesion of micro organism to host cells is at all times the primary, decisive step within the growth of infectious ailments. The goal of this adhesion by infectious pathogens is to colonize the host organism (e.g., the human physique) then set off an an infection, which might finish fatally within the worst case. A exact understanding of the micro organism’s adhesion to host cells is vital to discovering therapeutic alternate options that block this essential interplay within the earliest attainable stage of an an infection.
Critical interplay with the human protein fibronectin
In collaboration with different researchers, scientists from University Hospital Frankfurt and Goethe University Frankfurt have now defined the precise bacterial adhesion mechanism utilizing the human-pathogenic bacterium Bartonella henselae. This pathogen causes “cat-scratch illness,” a illness transmitted from animals to people. In a global collaborative mission led by the Frankfurt analysis group headed by Professor Volkhard Kempf, the bacterial adhesion mechanism was deciphered with the assistance of a mix of in-vitro adhesion checks and high-throughput proteomics. Proteomics is the examine of all of the proteins current in a cell or a posh organism.
In their examine revealed in Microbiology Spectrum, the scientists make clear a key mechanism: the bacterial adhesion to the host cells may be traced again to the interplay of a sure class of adhesins—referred to as “trimeric autotransporter adhesins”—with fibronectin, a protein typically present in human tissue. Adhesins are parts on the floor of micro organism that allow the pathogen to stick to the host’s organic constructions. Homologues of the adhesin recognized right here as essential are additionally current in lots of different human-pathogenic micro organism, such because the multi-resistant Acinetobacter baumannii, which the World Health Organization (WHO) has categorised as the highest precedence for analysis into new antibiotics.
State-of-the-art protein analytics have been used to visualise the precise factors of interplay between the proteins. In addition, it was attainable to indicate that experimental blocking of those processes virtually totally prevents bacterial adhesion. Therapeutic approaches that goal to stop bacterial adhesion on this means might symbolize a promising remedy different as a brand new class of antibiotics (referred to as “anti-ligands”) within the consistently rising area of multi-resistant micro organism.
New method to treating infectious ailments as an alternative choice to antibiotics
More info:
Diana J. Vaca et al, Interaction of Bartonella henselae with Fibronectin Represents the Molecular Basis for Adhesion to Host Cells, Microbiology Spectrum (2022). DOI: 10.1128/spectrum.00598-22
Provided by
Goethe University Frankfurt am Main
Citation:
How micro organism adhere to cells: Basis for the event of a brand new class of antibiotics (2022, June 30)
retrieved 30 June 2022
from https://phys.org/information/2022-06-bacteria-adhere-cells-basis-class.html
This doc is topic to copyright. Apart from any honest dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is supplied for info functions solely.