Graphical abstract. Credit: Journal of Membrane Science (2022). DOI: 10.1016/j.memsci.2022.121237
Hafnium (Hf) has no independent ore in nature; it is always closely symbiotic with zirconium (Zr) in a homogeneous form, and accounts for only approximately 2% of Zr.
Zr and Hf have similar physical and chemical properties. Their nuclear properties such as neutron absorption cross section are completely opposite. Additionally, Zr and Hf have similar outer electronic structures, and due to the shrinkage of the lanthanide series, the atomic radius and ionic radius differ only slightly. Therefore, the separation of Zr and Hf, especially the enrichment of Hf, is very difficult.
In a study published in Journal of Membrane Science, the research group led by Prof. Yang Fan from Fujian Institute of Research on the Structure of Matter of the Chinese Academy of Sciences developed an advanced ion-imprinted membrane (IIMs) separation system to achieve highly efficient separation and enrichment of Hf.
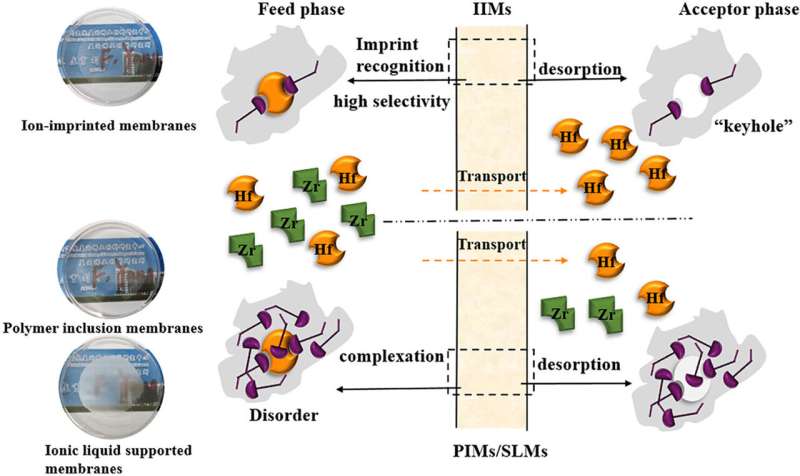
The researchers prepared ion-imprinted membranes (IIMs) using Hf ions as the imprinted ion, N, N-di-2-ethylhexyl diglycolamic acid (D2EHDGAA) as the carrier molecule, and cellulose triacetate (CTA) as the base polymer, and IIMs were applied to the separation and enrichment of Hf from zirconium oxychloride solution.
They found that IIMs can increase CHf/CZr from 2.33:100 to 33.33:100 within 1 h compared with polymer inclusion membranes (PIMs) and ionic liquid supported liquid membranes (SLMs), which increased by 3.33 and 3.67 times, respectively. The separation factor (SF) was increased by 1.13 and 1.40 times, respectively. The recovery rate of Hf was 29.2%, and separation-regeneration cycle experiments showed that the stability of IIMs was relatively good.
Additionally, the researchers revealed that IIMs, PIMs and SLMs can selectively separate and extract Hf ions in zirconium oxychloride solution, and the selectivity to Hf decreases in the order of IIMs > PIMs > SLMs. This selectivity is mainly attributed to the mutual structural matching between the imprinted holes of IIMs and Hf template ions and the complementary functional groups.
2023-02-13 17:00:03
Original from phys.org
The advancement of the membrane separation system has revolutionized the process of hafnium separation and enrichment, making it highly efficient and cost-effective. Hafnium, a hard transition metal, is used for a variety of applications, including nuclear power plants, aircraft, and electronics. It is also a crucial component for many newly emerging technologies such as spintronics, terahertz emitters, and others.
For successful industrial extraction and utilization of hafnium, it is essential to separate and enrich hafnium from a mixture of zirconium and titanium. The traditional method of this process involves chemical separations that can be slow, expensive, hazardous, and less efficient.
In order to address this issue, scientists have developed an advanced membrane separation system that is based on porous polymer membranes. This technology allows for hafnium to pass across the membrane, separate from the rest of the mixture, and enrich in a much shorter time span, making it highly efficient. Furthermore, the membrane filtration system requires considerably less energy and chemical resources, resulting in a much lower cost of operation.
This advanced membrane separation system could be particularly useful for nuclear power plants, which require large quantities of highly enriched hafnium with a purity of over 99%. Its efficient and cost-effective nature could be crucial for the advancement of nuclear science, leading to better safety protocols and increased performance of the plants.
The efficient and cost-effective nature of the membrane separation system could have a wide range of applications. It could prove invaluable for industrial processes related to hafnium extraction and enrichment, as well as many other technologies that involve separation of different elements. Going forward, the development and further optimization of this system could revolutionize the way we synthesize and utilize hafnium and other materials.
